There are no projects in the garbage can.
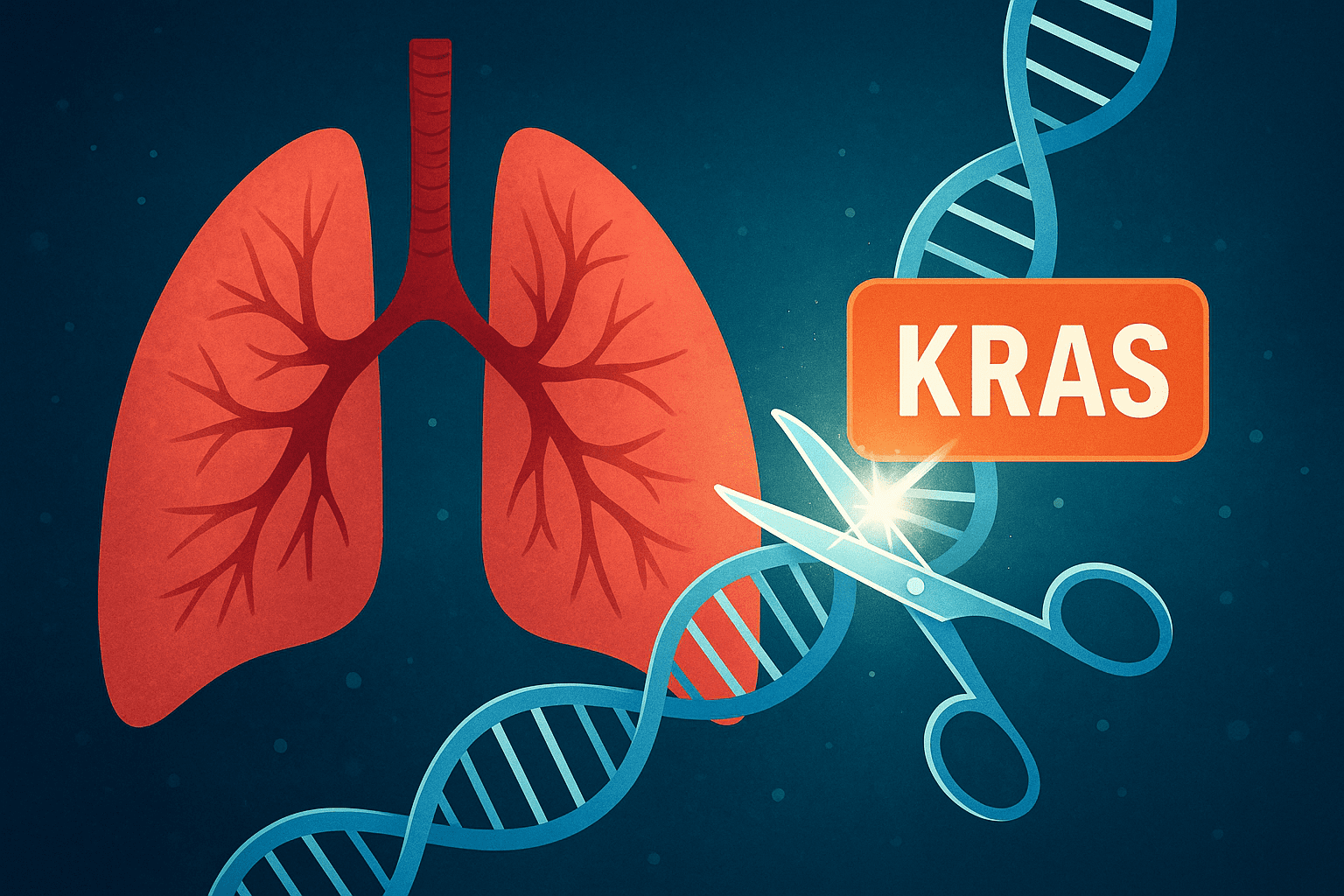
Researchers at GENyO have succeeded in directly eliminating the most common mutations of the KRAS gene from tumour DNA, which for decades was considered an ‘untouchable’ target in oncology. The strategy, tested in preclinical models of lung cancer, outperforms some of the current drugs in resistant cases and opens the door to more durable and personalised therapies.
Lung cancer remains the leading cause of cancer death worldwide. Every year, more than 30,000 new cases are diagnosed in Spain and, despite therapeutic advances, the five-year survival rate is barely above 20%. The problem is twofold: tumours are often detected at advanced stages and, when targeted treatments are applied, most quickly lose their effectiveness due to the development of resistance.
One of the main culprits is the KRAS gene, which acts as a molecular switch that tells cells when to grow and divide. When KRAS mutates, this switch gets stuck in the “on” position, causing uncontrolled proliferation. The result is aggressive tumours with a very poor prognosis. Approximately one-third of patients with lung adenocarcinoma have alterations in this gene.
For decades, KRAS has been known as an ‘untouchable gene.’ Numerous attempts to block its function failed, and for years it became the paradigm of oncogenes that were impossible to attack. The landscape changed in 2021 when the FDA approved sotorasib, the first inhibitor targeting the KRAS G12C mutation. Although the breakthrough was historic, it soon became clear that it was not the definitive solution: many patients do not respond to treatment and others develop resistance within months.
A multidisciplinary research group led by Pedro P. Medina, professor at the University of Granada and researcher at the GENyO centre, decided to explore a more radical approach. ‘Instead of blocking the protein already produced by the cell, we considered eliminating the mutation at its genetic source. This way, the tumour loses the basis for its growth,’ explains Dr. Medina.
High-precision genetic editing
The study, published in Nature Communications, describes how researchers have used a high-fidelity version of CRISPR-Cas9 known as HiFi-Cas9. This tool allows DNA to be cut with superior precision, minimising off-target errors that could damage healthy genes.
The scientists designed molecular guides capable of accurately identifying the mutated KRAS G12C and G12D variants, distinguishing them from the normal version of the gene. The challenge was to eliminate only the defective copies and leave the functional ones intact. ‘What is novel about our approach is that we were able to precisely and programmably inactivate the tumour’s genetic switch, targeting it specifically without affecting healthy cells,’ says Medina.
Promising results in preclinical models
The strategy was validated in different experimental settings. First, in two-dimensional and three-dimensional cell cultures, where gene editing drastically reduced the viability of tumour cells carrying mutated KRAS. Next, in patient-derived organoids, small mini-tumours grown in the laboratory that reproduce many of the characteristics of real cancer. In all cases, cell proliferation decreased significantly.
The team went further and tested the technique in patient-derived xenografts (PDX) in mice, a model considered more realistic. There, editing with HiFi-Cas9 significantly slowed tumour growth. In some cases, the efficacy was even higher than that of sotorasib. Most strikingly, it also worked in tumours that were previously resistant to the drug. ‘This suggests that CRISPR can overcome some of the resistance mechanisms that limit current treatments,’ says Dr Medina.
A key advantage over drugs
The essential difference with classic inhibitors is that CRISPR does not block the protein after its synthesis, but directly prevents its production. In this way, the tumour loses the mutation that causes its accelerated growth. This more profound intervention could explain why the strategy remains effective in contexts where drugs cease to work.
Caution and challenges ahead
Despite the promising results, the authors caution that this is still a proof of concept. The biggest challenge is finding a safe and efficient method to deliver the gene-editing tools to tumour cells within the human body. Viral particles were used as vehicles in this study, but this technology needs to be optimised before it can be considered for clinical trials.
‘It is essential to emphasise that we are not talking about a therapy that is ready for patients. We still need to figure out how to efficiently administer these tools and ensure their long-term safety,’ says Dr Medina.
A shared vulnerability
KRAS not only drives lung cancer, it is also involved in pancreatic and colon tumours, which are among the most lethal and have the worst prognosis. If tumour cells’ dependence on these mutations — what scientists call ‘tumour addiction’ — can be exploited as a vulnerability, the strategy could be extended to a broader spectrum of neoplasms.
In addition, the work opens up a pathway to explore how KRAS contributes to immune system evasion. Some evidence suggests that eliminating this mutation could improve the effectiveness of immunotherapies, although this aspect has yet to be confirmed.
A step towards personalised medicine
Genetic editing with HiFi-Cas9 represents a paradigm shift. It is not about blocking symptoms, but directly correcting the genetic error that causes the disease. In practice, this means moving towards more precise, longer-lasting and personalised therapies.
‘What we have demonstrated is that we can precisely and programmably switch off the malignancy of key genes that fuel tumours. This experimental therapy could inspire the development of much more personalised treatments in the medicine of the future,’ summarises Dr Medina.
International Impact
Various scientific media outlets have reported on the findings, including CRISPR Medicine News, which devoted an article to highlighting the selectivity and precision of the strategy developed (https://crisprmedicinenews.com/news/highlight-hificas9-selectively-disables-mutant-kras-in-lung-tumours/). In addition, the publisher Nature itself recently highlighted it among the most relevant articles in the field of cancer and the global impact of these results (https://l1nq.com/j81XI).
The collective effort
The project has been made possible thanks to the collaboration of multiple institutions: the University of Granada, the GENyO centre, the CNIO and the 12 de Octubre University Hospital (i+12), Madrid (Irene Ferrer and Luis Paz-Ares), the General University Hospital of Valencia and the Polytechnic University of Valencia (Eloisa Jantus-Lewintre).
The research was funded by the Spanish Association Against Cancer, the Ministry of Science and Innovation and the Regional Government of Andalusia, support that reflects the importance of investing in high-risk scientific projects with great translational potential.
“Lo que demostramos es que podemos apagar, de manera precisa y programable, la malignidad de genes clave que alimentan a los tumores. Esta terapia experimental podría inspirar el desarrollo de tratamientos mucho más personalizados de la medicina del futuro”, resume el Dr. Medina.
An open horizon
There is still a long way to go before this strategy can be applied to patients. Years of technological development and clinical validation will be required. However, the study marks a milestone by demonstrating that it is possible to eliminate key mutations directly from tumour DNA. In a field where advances often come in small doses, this approach represents a conceptual leap that could redefine the way certain cancers are treated.
‘The fact that we have been able to advance this therapy to in vivo models gives us confidence that gene editing tools could become real therapies in the future,’ concludes Dr Medina.
Reference:
High-fidelity Cas9-mediated targeting of KRAS driver mutations restrains lung cancer in preclinical models. Nature Communications (2025). doi:10.1038/s41467-025-62350-4
See more news